Osmotic Pressure 2 - Hyperosmotic, Isoosmotic, Hypoosmotic
Mr.Neuko! Please help me!
What's the matter?
In today's anatomy class, we had a lesson about the renal tubules, but I didn't understand it at all!
I had a hard time because I didn't really understand things like hyperosmotic and hypoosmotic.
I see. It seems the time has come to revisit the missed lectures.
Missed lectures?
Indeed. The remaining lessons on osmotic pressure.
Let me be clear. If you don't understand the upcoming explanations about the renal tubules, you won't be able to understand anything.
Is that so!?
However, if you listen to today's lecture, all your worries will be resolved! Therefore, listen attentively!
Thank you, I appreciate your help!
Today's Topic
Let's start with a review of the previous lecture.
Please go ahead.
We discussed osmotic pressure as the term for the movement of water.
Without delay, in this diagram, do you remember in which direction water moved, towards the side with salt or without?
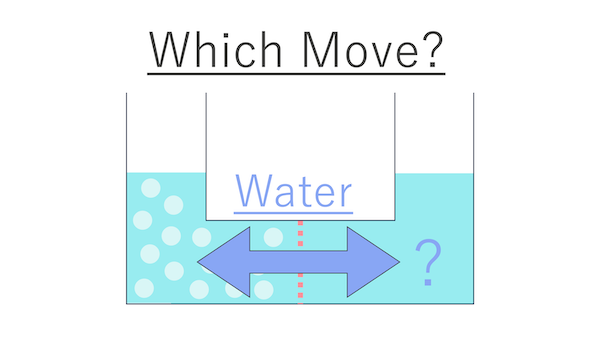
I remembered myself until yesterday.
I see. The correct answer is that water moves towards the side with salt, isn't it?
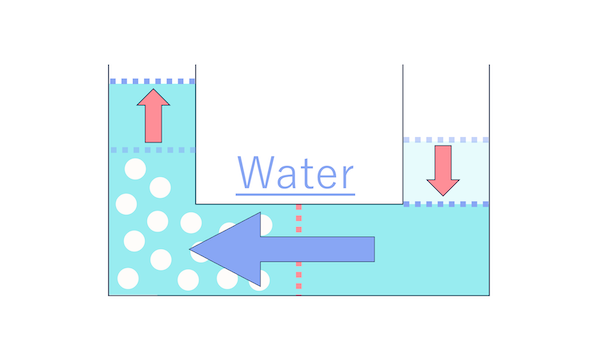
I remembered.
Good. Let's now proceed with today's lecture.
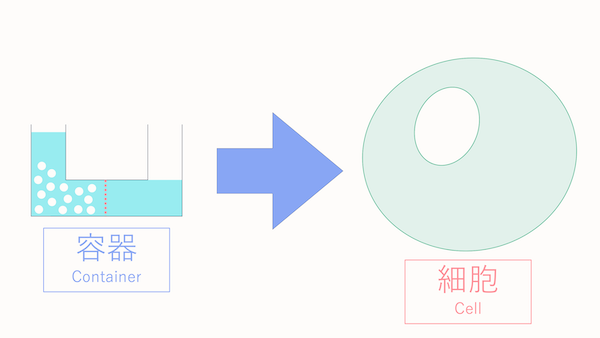
In the previous lecture, we explained the movement of water in a container to help you understand osmotic pressure.
However, in today's lecture, we will delve into the movement of water based on osmotic pressure within actual cells.
So, we're moving from containers to cells.
Exactly.
When explaining osmotic pressure within cells, there's no avoiding the terms
Hyperosmotic, Isoosmotic, and Hypoosmotic.
Those words sound quite complex.
Indeed. However, the concept itself is quite straightforward.
It simply refers to whether the concentration of the fluid surrounding the cell(extracellular fluid) is higher or lower compared to the fluid inside the cell (intracellular fluid).

I'm starting to understand it somehow.
Is that so?
Hyperosmotic
Let's begin with an explanation of hyperosmotic.
Please go ahead!
First, take a look at the diagram below.
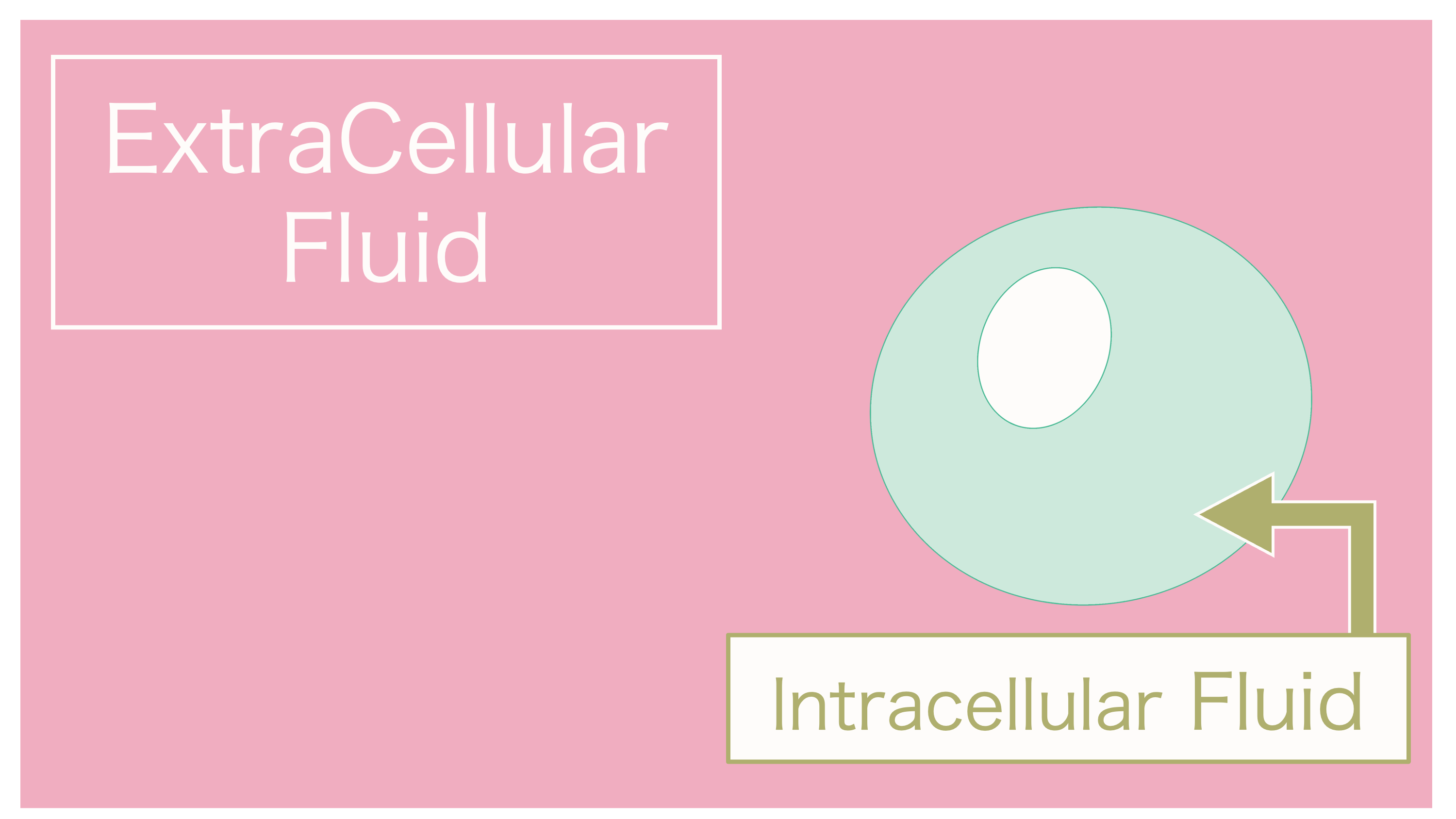
This diagram illustrates a cell residing within extracellular fluid.
Please take note that extracellular fluid is depicted in pink, and intracellular fluid is depicted in green, to enhance understanding.
It looks just like me, doesn't it?
It's just your imagination.
Let's assume that the fluid around the cell has become highly concentrated due to a large amount of salt and other substances.
If we observe this for a while without any changes, what do you think will happen to the water inside the cell?
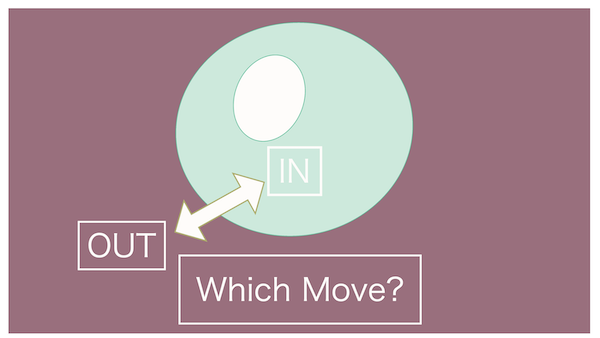
In the earlier review, the teacher mentioned that water moves towards the more concentrated side.
So, does that mean the water inside the cell will move out?
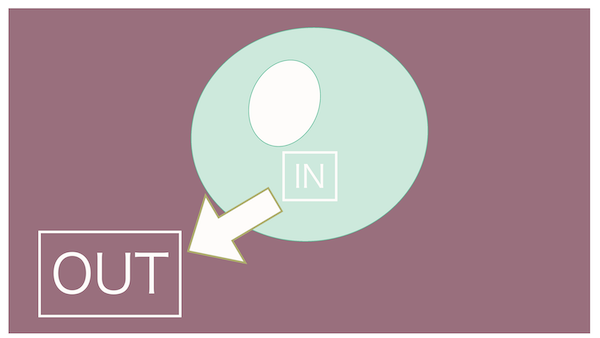
Absolutely right!
Oh!
Just as Cellnosuke, water ends up being pulled out of the cell.
To elaborate further, if the concentration of extracellular fluid is higher than that of intracellular fluid,
intracellular fluid will move to extracellular fluid through osmosis.
Teacher, is it okay for the cell to lose water?
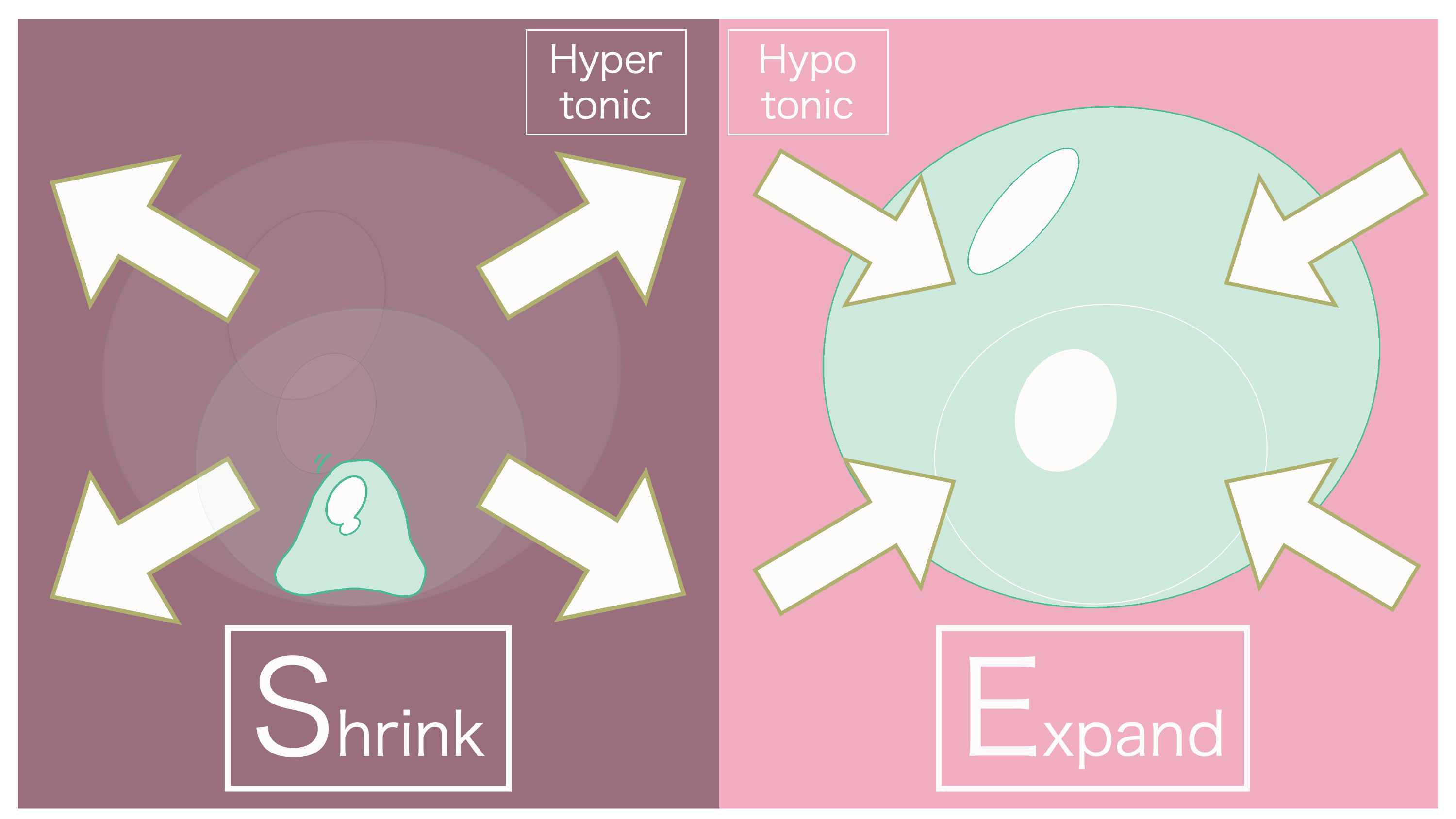
A great question. In this case, the cell will shrink as intracellular fluid decreases.
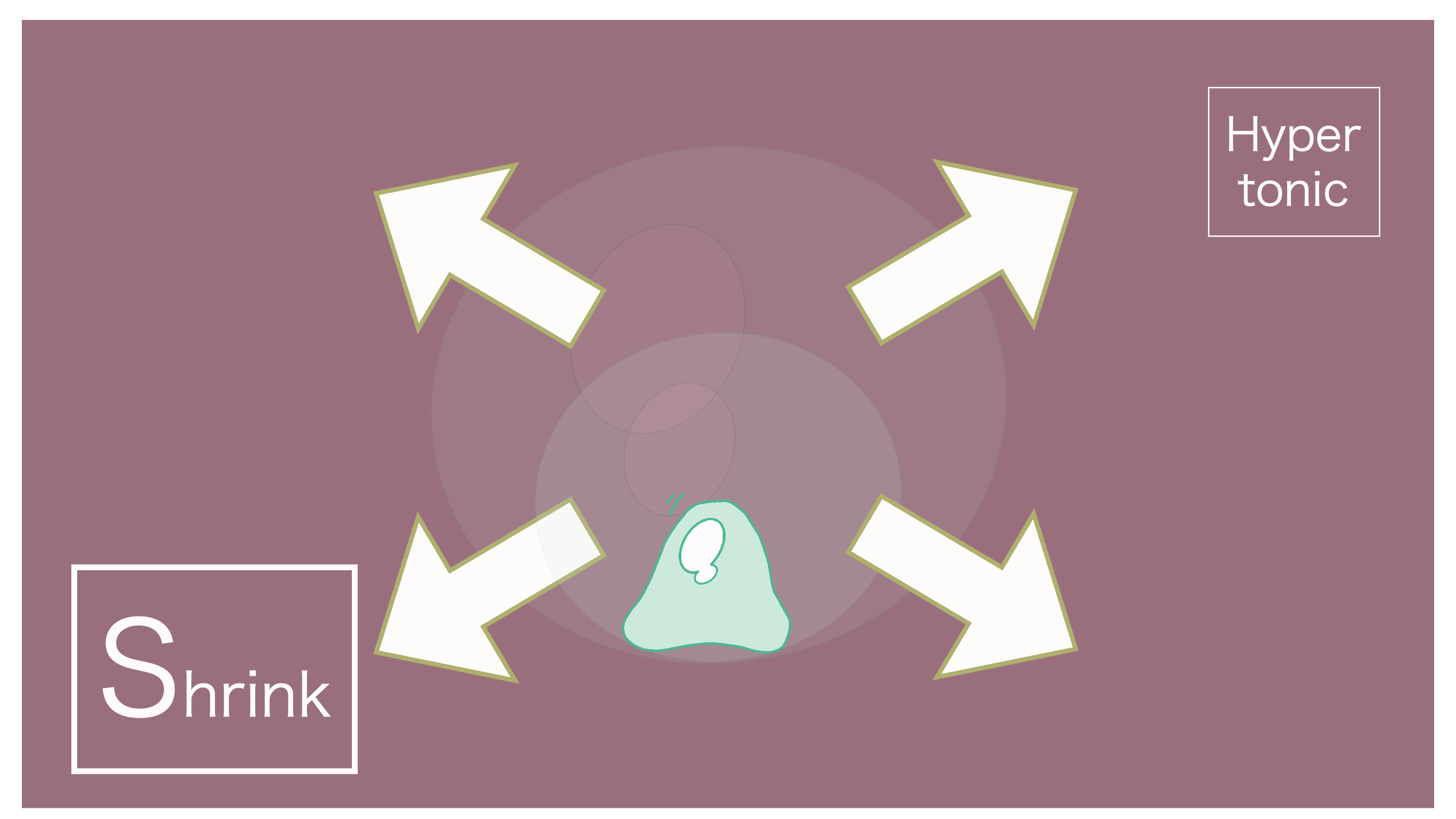
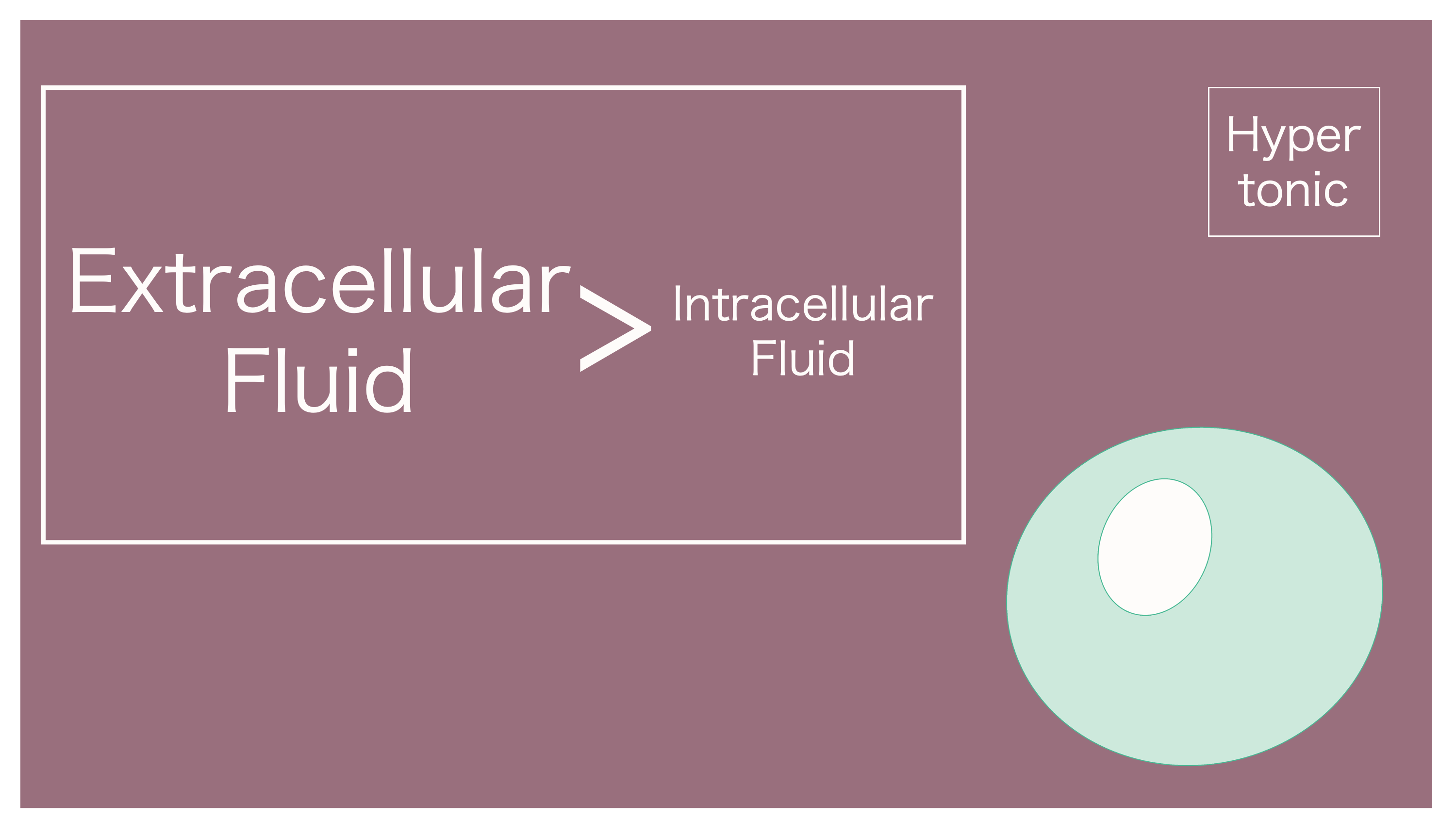
And thus, the osmotic pressure that occurs when extracellular fluid is concentrated is referred to as "hyperosmotic."
I became a bit confused with the explanation of hyperosmotic.
That might be the case. To help you grasp the concept better, let me rephrase it:
Hyperosmotic refers to when the concentration of extracellular fluid is greater (higher) than that of intracellular fluid.
How about this explanation?
It's become easier to visualize!
Including the explanations that follow, understanding becomes simpler when you consider whether extracellular fluid is concentrated or dilute.
It's "hyperosmotic" because extracellular fluid is more concentrated. Water within the cell moves outwards.
Hypoosmotic
If you've grasped hyperosmotic correctly, the rest is straightforward.
Please continue.
This time, it's the reverse of what we discussed earlier.
Let's assume that extracellular fluid loses a significant amount of salt, becoming less concentrated than intracellular fluid.
If we observe this for a while without any changes, what do you think will happen to the water inside the cell?
This time, the extracellular fluid is less concentrated, right?
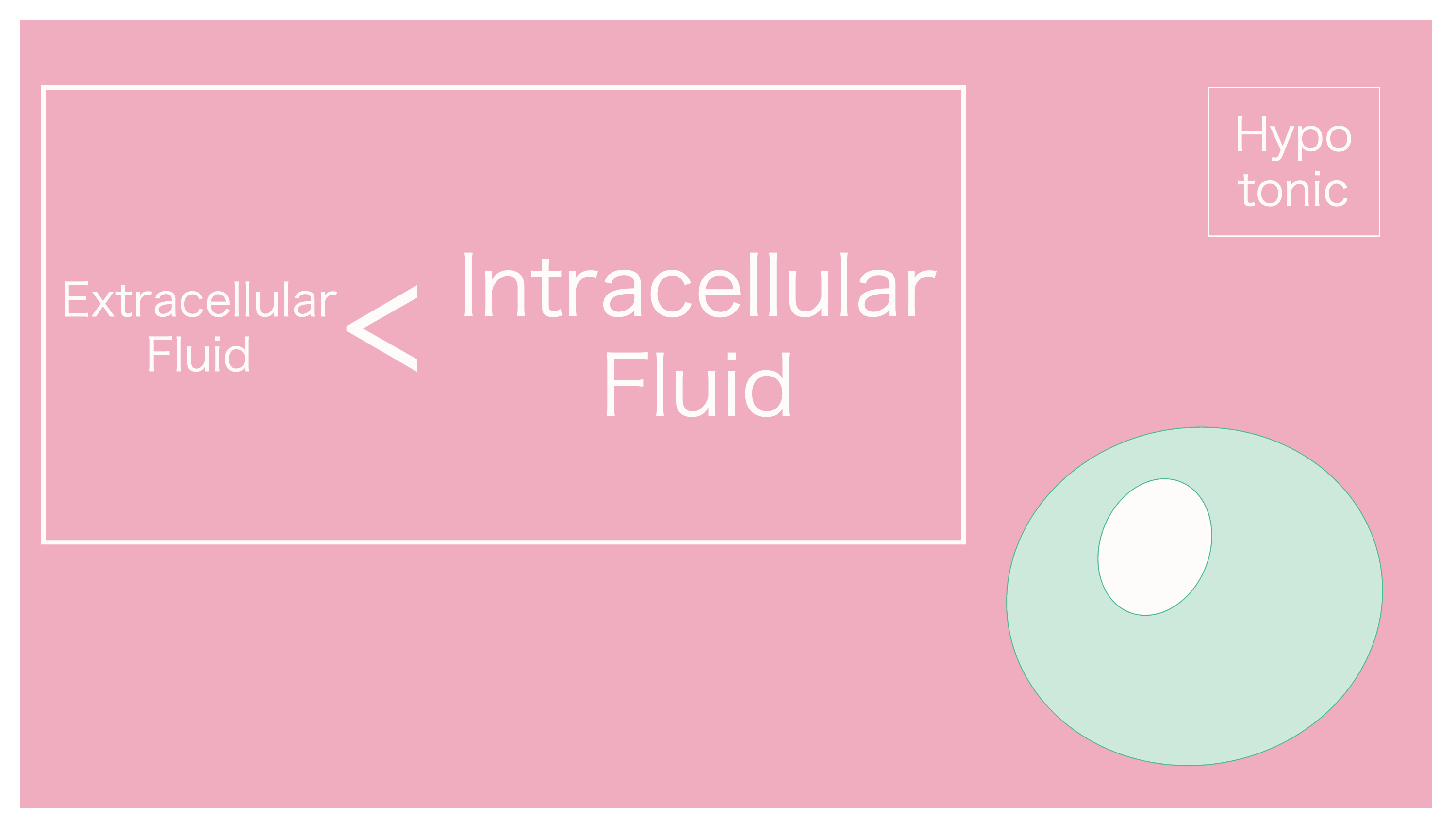
If that's the case, I believe water would enter the cell.
Could it be that the dilute side (extracellular fluid) is trying to dilute the concentrated side (intracellular fluid)?
Absolutely right!
Yes! I got it!
Just as Cellnosuke, in this case, extracellular fluid flows into the intracellular fluid in the opposite direction.
As a result, it's good to keep in mind that the cell can swell up due to the influx of water.
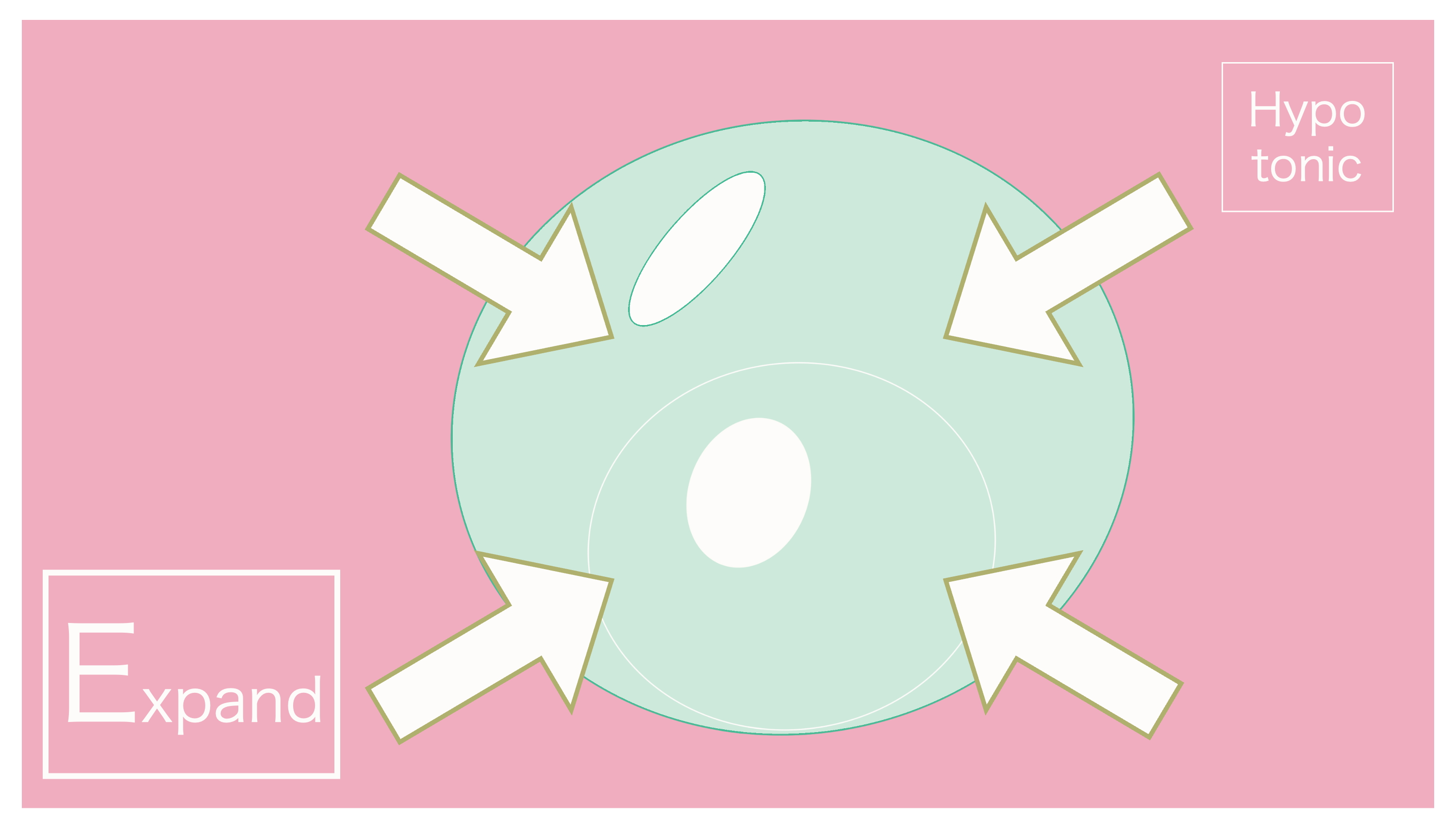
And thus, due to the extracellular fluid having a lower concentration (being dilute), the osmotic pressure that occurs during this time is referred to as "hypoosmotic."
It's "hypoosmotic" because extracellular fluid is dilute. Water moves from extracellular fluid into the cell.
Isoosmotic
By now, you've come far enough that just hearing "isoosmotic" should give you an idea of what it means, right?
Does "isoosmotic" mean that the concentration of intracellular fluid and extracellular fluid is the same (equal)?
Correct!
Wow!
Continuing with the questions, in this case, which way will the water move?
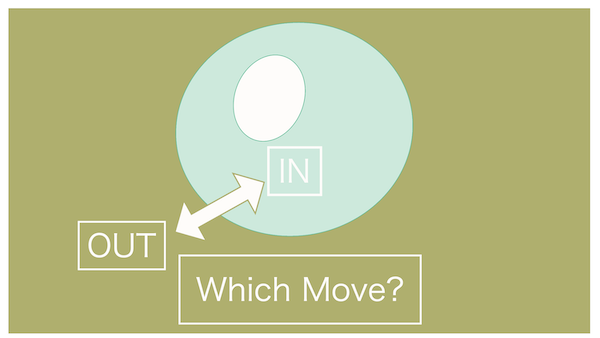
Does that mean it won't move this time...?
You're on fire today! That's correct!
Ehehe.
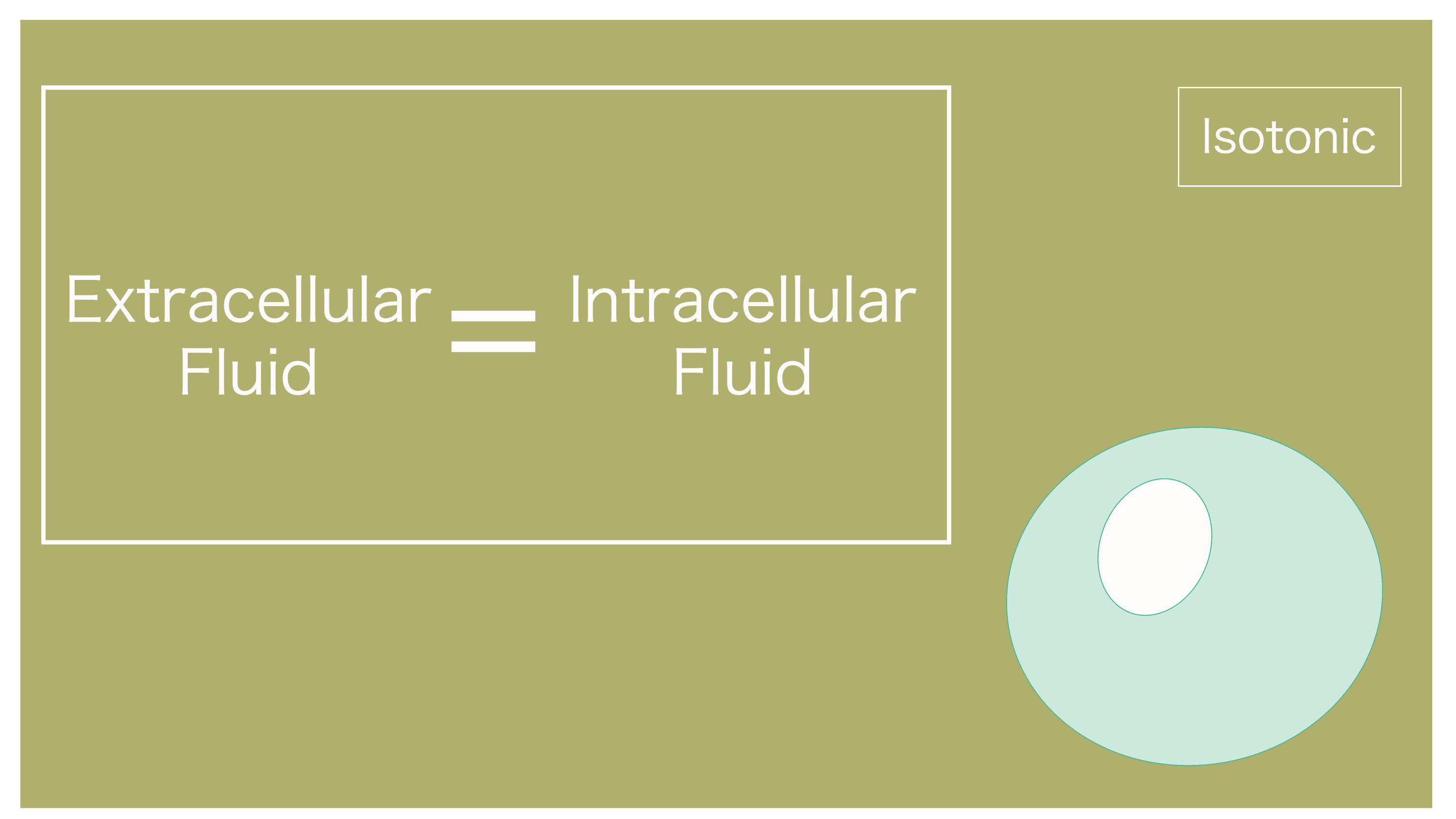
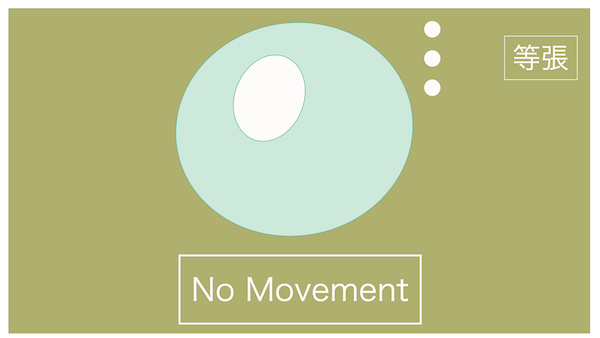
To summarize, when the concentration of the extracellular fluid and intracellular fluid is the same, it's called isotonic.
Since the concentrations are the same, naturally, there won't be any movement of water.
Extracellular and intracellular fluids have the same concentration, hence isotonic. No water movement.
Supplement: Osmotic Homeostasis
Teacher, I have a question.
What's the matter?
Both hypertonic and hypotonic conditions, where cells shrink or swell, wouldn't be good for the cell's health, right?
You noticed that well. Impressive growth.
Ehehe.
When the area around a cell becomes hypertonic and excessive water is drawn out, it puts the cell's life at risk.
If it happens to just one cell, that's one thing, but if it occurs in all the cells of the body, it's a catastrophe.
This is where the concept of Homeostasis (maintaining stability) comes into play.
Homeostasis...?
Yes.
Mainly through the use of the kidneys, the body maintains osmotic pressure by regulating the amount and concentration of water.
So the kidneys come into play here!
Exactly. Of course, temporary disturbances in osmotic pressure due to dietary habits and such can burden cells.
However, the cells work to ultimately restore osmotic pressure to a normal range.
This kind of function is referred to as Homeostasis, the maintenance of stability.
I feel like things are connecting now!
Isn't that right? Anatomy is a subject where the more you study, the more it connects to the things you've learned before.
And the more knowledge connects, the more enjoyable it becomes, doesn't it?
Therefore, keep studying steadily and don't give up.
Yes!
With that, we'll conclude today's lecture.
Thank you very much!
Lastly, let's go through a problem.

Water moves from the intracellular fluid to the extracellular fluid (cell shrinks).

Water moves from the extracellular fluid to the intracellular fluid (cell expands).

No movement