Osmotic Pressure 1
I didn't understand anything in today's physiology class. I wonder what osmotic pressure is after all.
But, I'm sure it'll be alright. Let's go home and play some games today.
You're naive, Cellnosuke!
M-Mr. Neuko!
Let me be clear. Physiology becomes quite dull if you don't understand osmotic pressure.
I-Is that so!?
In the future, when you learn about kidney function or study the pathophysiology of edema, knowledge of osmotic pressure will be essential.
Do you want to attend classes without understanding anything?
No, I don't want that.
Is that so, huh?
On the other hand, if you can understand osmotic pressure, physiology will undoubtedly become enjoyable.
Wow...!
Alright then, let's begin today's lecture! Listen attentively!
Thank you, I will!
The Name is Osmotic Pressure
First of all, do you know what diffusion is referring to?
I really don't understand at all.
I see. Osmosis refers to the method of water movement within cells.
Water movement?
Yes.
You can roughly interpret it as the method cells use to regulate their water content for survival.
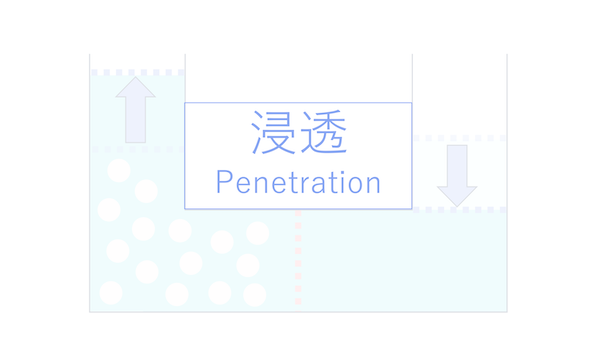
And as a result of cells regulating water through osmosis, the pressure that arises is called osmotic pressure.
How about it? Do you understand a little now?
Other than the name of the water movement method, I still don't understand at all.
I see. I will explain step by step.
Osmosis is the method of water movement. Osmotic pressure refers to the pressure that arises as a result of this movement.
What is Osmotic Pressure
Now, let's explain osmosis specifically.
Please do.
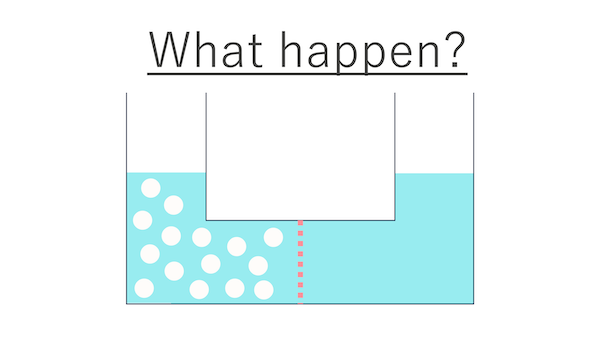
First, take a look at this diagram.
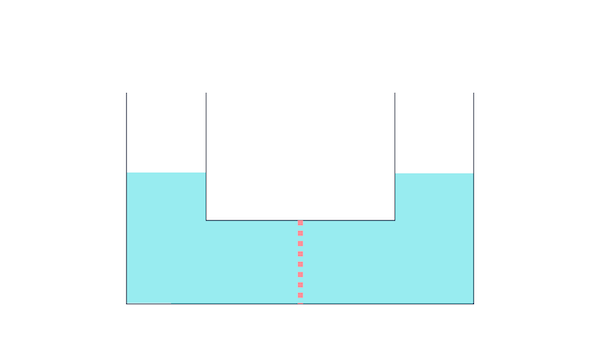
The container has water on both sides, right?
What is the dashed line in the middle?
The dashed line represents a semipermeable membrane.
Semipermeable membrane?
Look back at the previous lecture. For now, just understand that it's a partition with holes.
Let's continue the lecture.
Yes.
Let's imagine that we have put a large amount of salt only on the left side of this container.
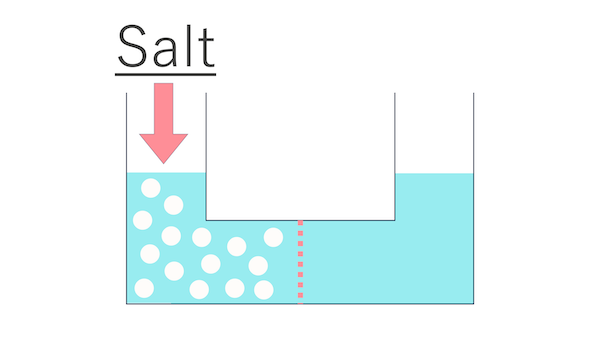
Salt has been added to the water on the left side.
I see. And let's assume that water can freely pass through the holes of the partition, but salt cannot.
Now, I have a question for Cell-kun.
What is it?
If we leave this container alone for a while, what do you think will happen to the water and the salt?
Hmm... I have no idea at all.
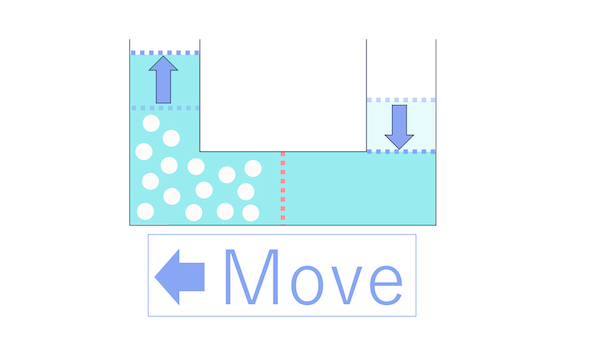
Since salt can't move, only water will move to one side, right?
Correct!
Huh?
That's right, water moving is the correct answer.
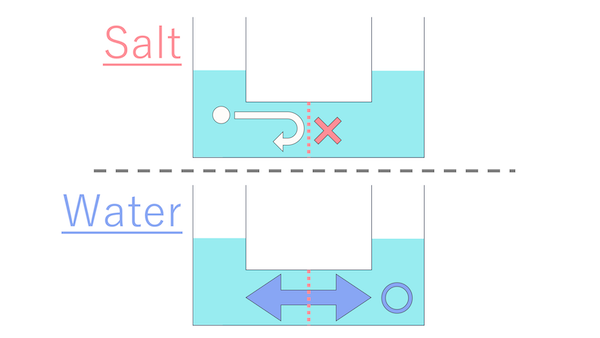
Now, between the side with salt and the side without, which way do you think water will move?
To the side without salt?
Close, but not quite! The correct answer is that water will move to the side with salt.
...Does that mean water is attracted to the concentrated side?
You're on fire today! That's absolutely correct!
Hehehe.
As Cell-kun mentioned,
when you divide a container filled with water using a partition with holes, water moves from the side with a higher concentration to the side with a lower concentration.
This phenomenon is called osmosis. By the way, in the case of cells, the partition is the cell membrane.
I think I can imagine it a little!
That's the spirit! And as a result of osmosis moving the water, do you think the side without salt would have less water now?
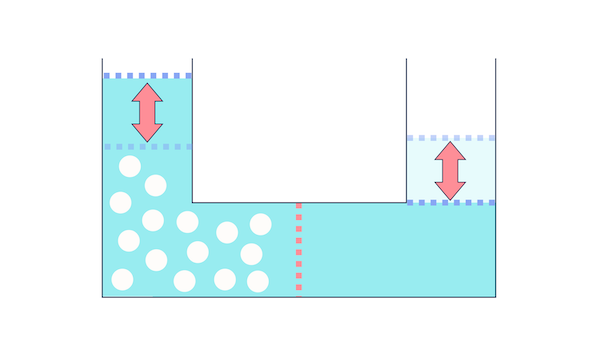
Yes, it has decreased.
The pressure difference that arises from this movement is called osmotic pressure.
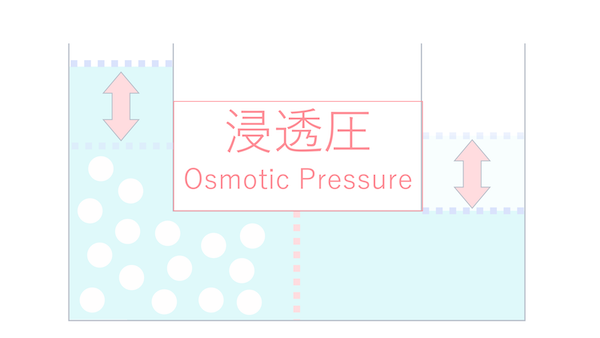
I don't quite understand osmotic pressure.
In the scope of Cell-kun's physiology class, there's no need to explain in such detail.
In reality, the terms "osmosis" and "osmotic pressure" are often used interchangeably in physiology classes.
So, understanding that osmosis refers to the movement of water from a concentrated area to a diluted area should be sufficient.
Lastly, remember that osmosis is a form of passive transport that does not require energy.
Understood!
Osmosis (osmotic pressure) refers to the movement of water from a concentrated area to a diluted area.
Osmosis (osmotic pressure) is a form of passive transport.
Now, let's conclude today's lecture here.
Is that all for today?
Osmotic pressure is an essential concept in the study of physiology, and it includes explanations about hypertonic, isotonic, and hypotonic conditions that cannot be avoided.
Since the lecture would become lengthy, we decided to split it into two parts.
I see, that makes sense.
To better understand the upcoming discussion about hypertonic, isotonic, and hypotonic conditions, it's important to solve problems and review thoroughly.
Understood!

To the side with salt
Osmosis (osmotic pressure)
No, it doesn't require energy (passive transport)